Loading... Please wait...
Loading... Please wait...- Home
- Anti Estrogens
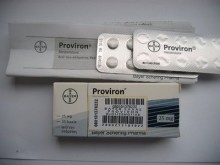
- Proviron (mesterolone) 25mg by medipharm / Strip
Product Description
PROVIRON TABLETS
SCHEDULING STATUS:
S5
PROPRIETARY NAME
(and dosage form):
PROVIRON TABLETS
Oral androgen for the treatment
of male patients
COMPOSITION
1 tablet contains mesterolone (17beta-hydroxy-1alpha-methyl-5alpha-androstan-3-one) 25 mg and the preservatives methylparaben (0,02%) and propylparaben (0,01%).
PHARMACOLOGICAL CLASSIFICATION
A. 21.7 Male sex hormones.
PHARMACOLOGICAL ACTION
Proviron balances a deficiency of androgen formation which begins to fall gradually with increasing age. Therefore, Proviron is suitable for treatment of all conditions caused by deficient endogenous androgen formation. In the recommended therapeutic dosage, Proviron will not impair spermatogenesis. Proviron is especially well tolerated by the liver.
INDICATIONS
• Declining physical activity and mental alertness in middle- and old-aged men
Reduced efficiency, easy fatigability, lack of concentration, weak memory, disturbances of libido and potency, irritability, disturbances of sleep, depressive moods, and general vegetative complaints are often attributed to androgen-deficiency. These complaints can be overcome or improved by the use of Proviron tablets.
• Potency disturbances
Proviron overcomes potency disturbances due to androgen-deficiency. It may also be of use as supplementary therapy in cases of diminished potency where androgen-deficiency is not the primary cause.
• Hypogonadism
Growth, development and function of androgen-dependent target organs are stimulated by Proviron. It promotes development of secondary male sex characteristics in cases of prepuberal hypogonadism. Full clinical and laboratory investigations are necessary in all cases of young patients prior to commencement of treatment. Proviron tablets may also be used as a substitution therapy in cases where a loss of gonadal function has occurred post-puberally.
• Infertility
Oligozoospermia and deficient Leydig-cell secretion may be the cause of infertility. With Proviron treatment, sperm count can be increased, the quality improved and, furthermore, a higher fructose concentration up to normal values can be achieved thus increasing the chances of procreation.
CONTRA-INDICATIONS
In patients with carcinoma of the prostate, androgen therapy of any kind, including the use of Proviron, is contra-indicated.
DOSAGE AND DIRECTIONS FOR USE
If not otherwise directed by the physician, the following dosage is recommended:
In declining physical activity and potency disturbances
Commencement of treatment: 1 Proviron tablet of 25 mg three times daily.
Continuation of treatment: 1 Proviron tablet of 25 mg twice or once daily.
According to type and severity of the complaints, a course of Proviron lasting four to six weeks or a prolonged uninterrupted treatment over several months is recommended. If required, the course of treatment may be repeated several times.
Hypogonadism requires continuous therapy
For development of secondary male sex characteristics 1 Proviron tablet of 25 mg 3-4 times daily for several months. As maintenance dose 1 Proviron tablet of 25 mg twice or three times daily will be sufficient.
In oligozoospermia
1 Proviron tablet of 25 mg twice or three times daily for a cycle of spermatogenesis, ie 90 days.
In case of simultaneously impaired gonadotrophic excretion, a combined therapy with gonadotrophic hormone exhibiting FSH activity is recommended for the commencement of treatment (eg 2000 IU serum gonadotrophin im twice weekly up to a total amount of 12 000 IU). If necessary, Proviron treatment is to be repeated after an interval of several weeks.
SIDE EFFECTS AND SPECIAL PRECAUTIONS
The administration of Proviron is recommended only for male patients.
Regular examinations of the prostate should be carried out prophylactically.
KNOWN SYMPTOMS OF OVERDOSAGE AND PARTICULARS OF ITS TREATMENT
None.
IDENTIFICATION
Small, white, round tablets with flat sides and bevelled edges. Upper side: impressed with AX in an equilateral hexagon. Lower side: scored.
PRESENTATION
20 and 100 tablets.
STORAGE INSTRUCTIONS
Store below 30°C. For shelf-life, refer to the imprint on the pack. Keep out of reach of children.
REGISTRATION NUMBER
B/21.7/137
NAME AND BUSINESS ADDRESS OF THE APPLICANT
Schering (Pty) Ltd
(Reg No: 1964/009072/07)
106 Sixteenth Road
Randjespark
Midrand 1685
P O Box 5278
Halfway House 1685
DATE OF PUBLICATION OF THIS PACKAGE INSERT
6 February 1979
SCHERING (PTY) LTD
(Reg No: 1964/009072/07)
Subsidiary of
Schering AG Germany
Updated on this site: April 2003
Current: January 2005
Source: Pharmaceutical Industry
Customers Who Viewed This Product Also Viewed
Currency Converter
Choose a currency below to display product prices in the selected currency.